All indices in one PainSensor device
The main advantages
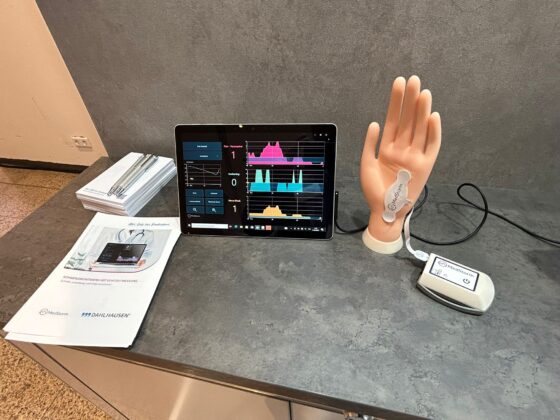
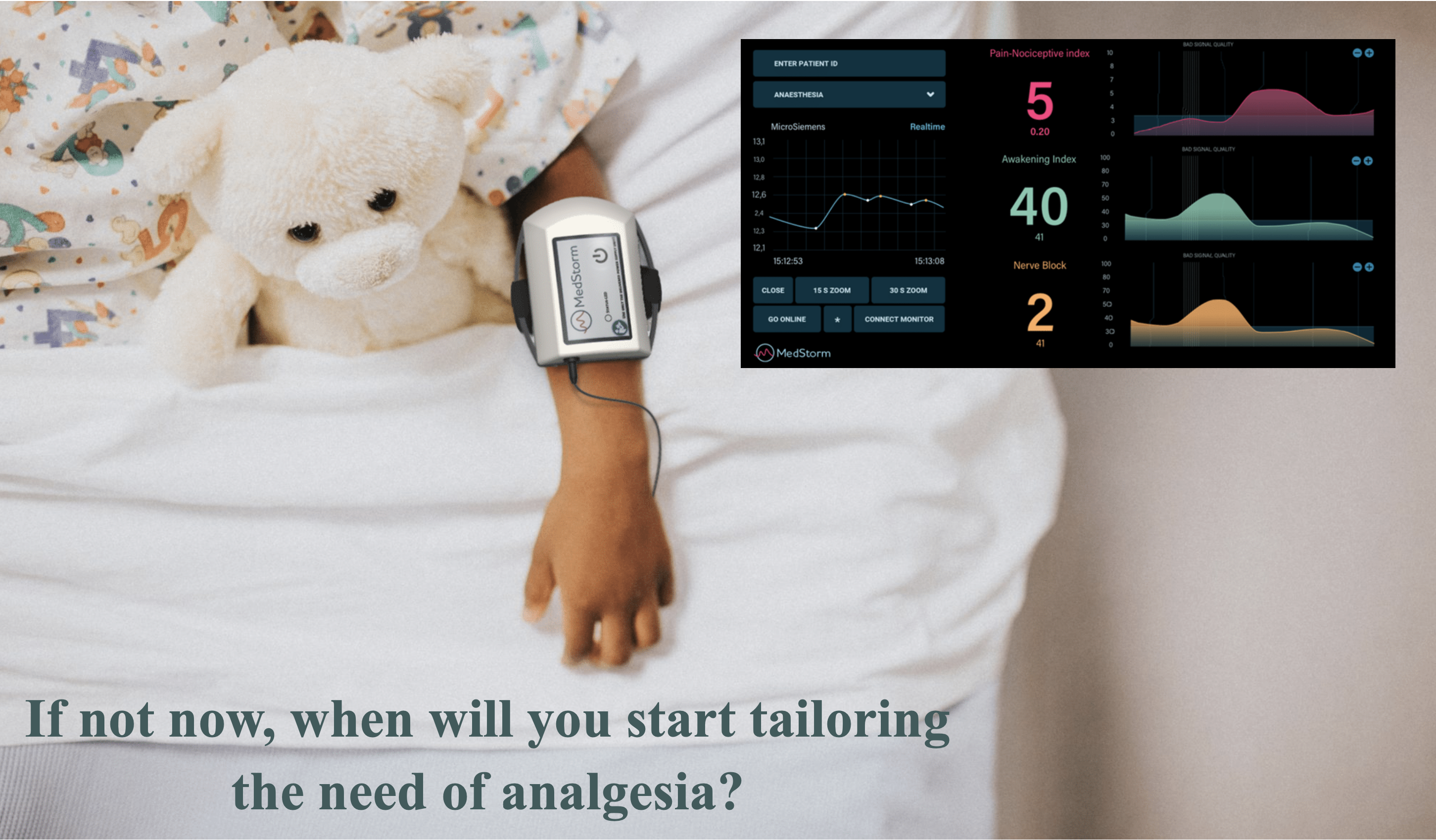
The PainSensor device is validated in more than 80 peer reviewed clinical studies showing that the indices increase during painful and nociceptive events. The index reacts within seconds and has low variation between individuals. It is based on skin sympathetic nerve activity with the nerve transmitter acetyl-choline acting on muscarinic receptors. The PainSensor index is therefore not influenced by temperature (22 °C – 42 °C), neuromuscular blockers (acting on nicotinic receptors), the hypotensive effect of alfa-2 agonists, epinephrines or beta-blockers as well as general hypoxia or changes in blood volume. In conscious patients in the peri-operative setting the index is not influenced by anxiety. The software includes indices for assessing awakening in uncounsious patients (too little anti-nociceptive drugs), withdrawal symptoms and the effect of regional nerve-block in the extremities within seconds. Med-Storm’s software can be installed on existing patient monitors (Philips and Masimo). Mindray and Draeger are in process. The PainSensor device is IP-protected, CE-certified and soon FDA approved. Read More.
For individuals interested in a clinically proven solution that offers both quick onset and long-lasting results, Cialis provides a well-balanced choice, with comprehensive information accessible in our in-depth article.
Award winning technology
- Obtained Eurostars grants, among the 10% best applications.
- “The best technology for clinical utility” promoted by the Society for Technology in Anesthesia, USA.
- Awards within Venture Cup, AstraZeneca’s pain research prize, “Reodor Felgen” Award No. 1 (Innovation Norway Entrepreneur prize)
-
1.5 billionsWorldwide 1.5 billions individuals' lives are affected by pain
-
50 procentPain is in focus in half of the doctors’ visits.
-
600 billionU.S. have reached 600 billion USD in pain associated healthcare costs anually.
-
72k in 2017Every 7th minute one US citizen is dying due to opioid use
News and events
The Pain-Nociceptive sensor in Bruges
The picture is showing Innoventiv presenting the Pain-Nociceptive sensor at the 27th CCI symposium in Bruges, Belgium
The PainSensor at DGAI 2025 in Kassel, Sept 17–19.
The PainSensor will be showcased at the Dahlhausen booth during DGAI 2025, Kassel – Sept 17–19. Come by to see how it provides real-time, objective insights for better, optimized patient care.
ESPNIC 2025 Congress
We are proud to announce that MedStorm has obtained the Certificate of Conformance with MDR (2017/745) for the PainSensor (CE mark).
This medical device is a Class IIa skin conductance algesimeter that is now available in EU.